39 open label clinical trial
Open-Label Trial - an overview | ScienceDirect Topics In open label trials, both the study participant (and caregiver where applicable) and the investigator are aware of the treatment provided to study participants. A common use for this design is in an extension trial, which immediately follows a randomized controlled trial, and is used for gathering safety data in the same subjects. Open-label extension studies: do they provide meaningful information on ... Most of the value accrued in open-label extension studies is gained from a refinement in the perception of the expected incidence of adverse effects that have most likely already been identified as part of the preclinical and clinical trial programme.
Adaptive Designs for Clinical Trials of Drugs and Biologics based on a non-comparative analysis even in open-label trials, but ensuring that the adaptations are completely unaffected by knowledge of comparative data presents additional challenges.
Open label clinical trial
An open label randomized clinical trial of Indomethacin for mild and ... An open label randomized clinical trial of Indomethacin for mild and moderate hospitalised Covid-19 patients Authors Rajan Ravichandran 1 2 , Surapaneni Krishna Mohan 3 , Suresh Kumar Sukumaran 4 , Devakumar Kamaraj 5 , Sumetha Suga Daivasuga 6 , Samson Oliver Abraham Samuel Ravi 7 , Sivakumar Vijayaraghavalu 2 8 , Ramarathnam Krishna Kumar 9 Open-Label Trial - an overview | ScienceDirect Topics open-label trials of desipramine, tranylcypromine, reboxetine, and bupropion showed improvement with the drug therapy.55 an open-label trial of escitalopram for sad patients ( n = 20) over 8 weeks produced a response rate of 95% (sigh-sad < 50% of baseline value) and a remission rate (sigh-sad score < 8) of 85%. 56 in an open-label trial of … What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation.
Open label clinical trial. (PDF) What is an open label trial? - ResearchGate Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such. as a trial of devices, or psychological and physical ... Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play a key... NCI Dictionary of Cancer Terms Clinical Trials Information. A to Z List of Cancer Drugs. Complementary & Alternative Medicine (CAM) Questions to Ask about Your Treatment. Research. Coping with Cancer. Feelings and Cancer. Adjusting to Cancer. Self-Image & Sexuality. Day-to-Day Life. Support for Caregivers. Survivorship. Questions to Ask About Cancer.
Understanding Clinical Trial Terminology: What is a Long-Term Extension ... Clinical trials are important research studies that are conducted to test potential new medicines in people after earlier stages of research have been successfully completed. Clinical trials typically move forward in a series from early-stage, small-scale Phase 1 studies to late-stage, large-scale, Phase 3 studies. These studies could be double-blind in which case the trial […] An open label, multicenter clinical trial that investigated... : Medicine The aims of this prospective, open label, multicenter clinical trial were to establish its efficacy and safety during long-term use. Methods: Patients, who were all children, were treated with 1.88 to 3.75 mg leuprorelin subcutaneously once every 4 weeks for a total of 96 weeks between 2015 and 2018. Open-label Clinical Trial of Niraparib Combined With ... - JAMA Design, Setting, and Participants This open-label, single-arm, phase 2 study enrolled 55 eligible patients with advanced or metastatic TNBC irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression at 34 US sites. Data were collected from January 3, 2017, through October 29, 2018, and analyzed from October 29, 2018 ... What is an open label extension study? • NCK Pharma Open-label trials may be appropriate for comparing two very similar treatments to determine which is most effective. An open-label trial may be unavoidable under some circumstances, such as comparing the effectiveness of a medication to intensive physical therapy sessions. About Post Author admin (41) (10) (10)
What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation. Open-Label Trial - an overview | ScienceDirect Topics open-label trials of desipramine, tranylcypromine, reboxetine, and bupropion showed improvement with the drug therapy.55 an open-label trial of escitalopram for sad patients ( n = 20) over 8 weeks produced a response rate of 95% (sigh-sad < 50% of baseline value) and a remission rate (sigh-sad score < 8) of 85%. 56 in an open-label trial of … An open label randomized clinical trial of Indomethacin for mild and ... An open label randomized clinical trial of Indomethacin for mild and moderate hospitalised Covid-19 patients Authors Rajan Ravichandran 1 2 , Surapaneni Krishna Mohan 3 , Suresh Kumar Sukumaran 4 , Devakumar Kamaraj 5 , Sumetha Suga Daivasuga 6 , Samson Oliver Abraham Samuel Ravi 7 , Sivakumar Vijayaraghavalu 2 8 , Ramarathnam Krishna Kumar 9



























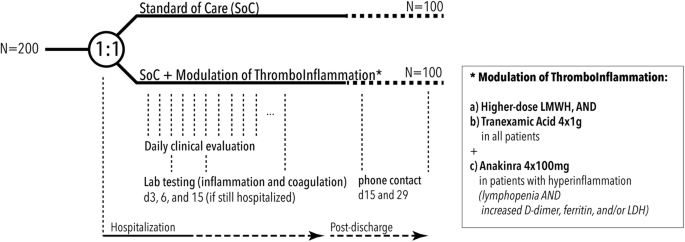


,%20Arthritis:%0A%0AIn%20open-label%20phase:%20treatment%20with%20tofacitinib%20for%20Arthritis.png?md=1)

Post a Comment for "39 open label clinical trial"