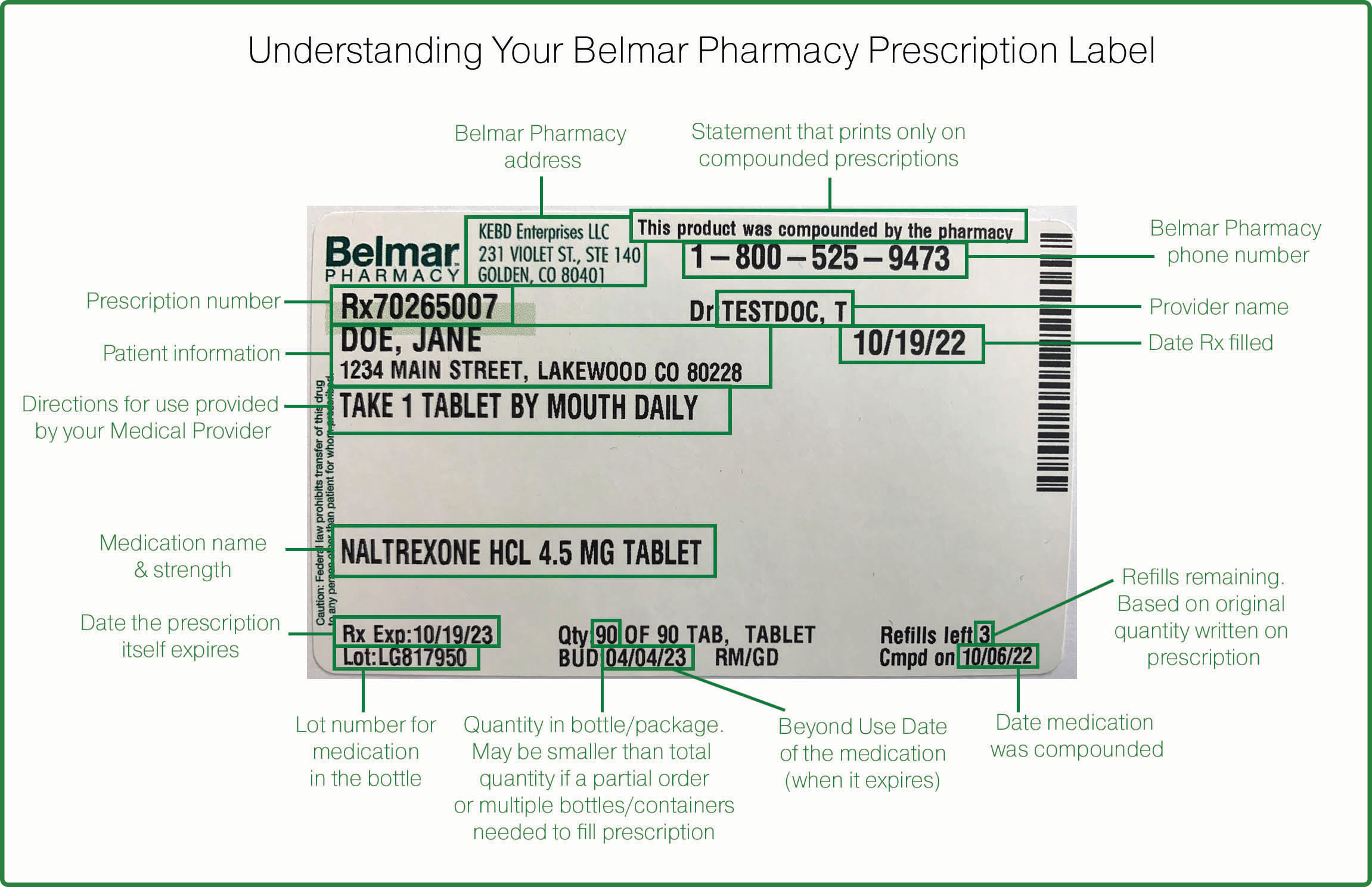
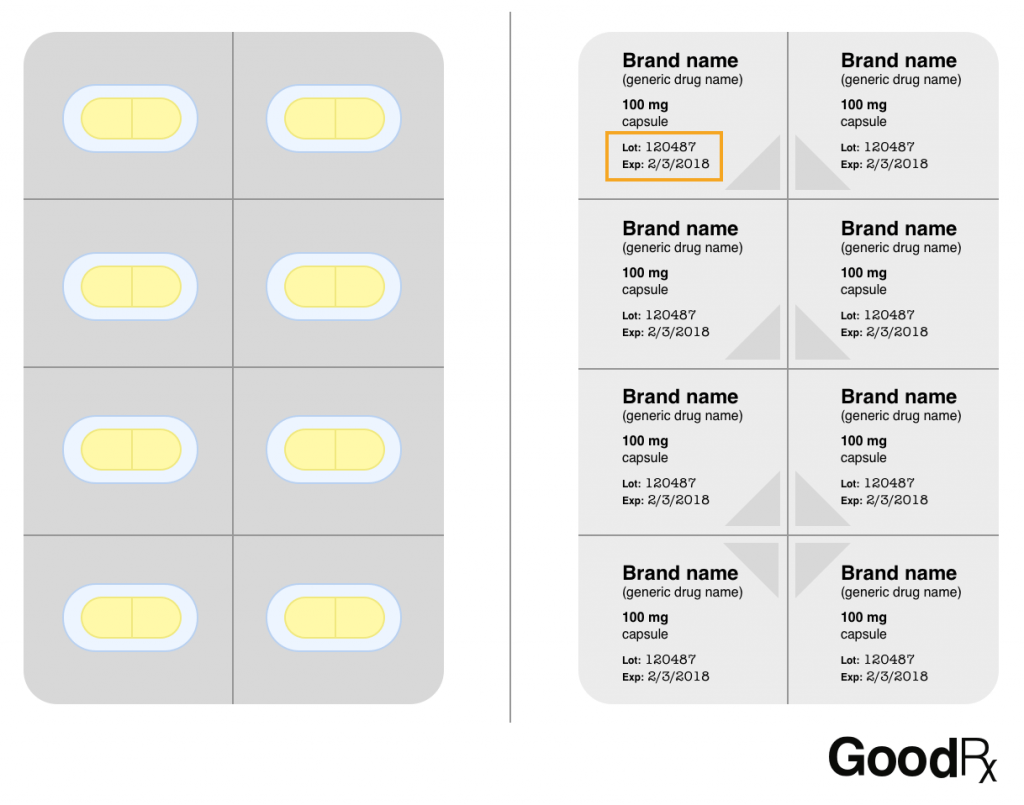
45 lot number on medication label
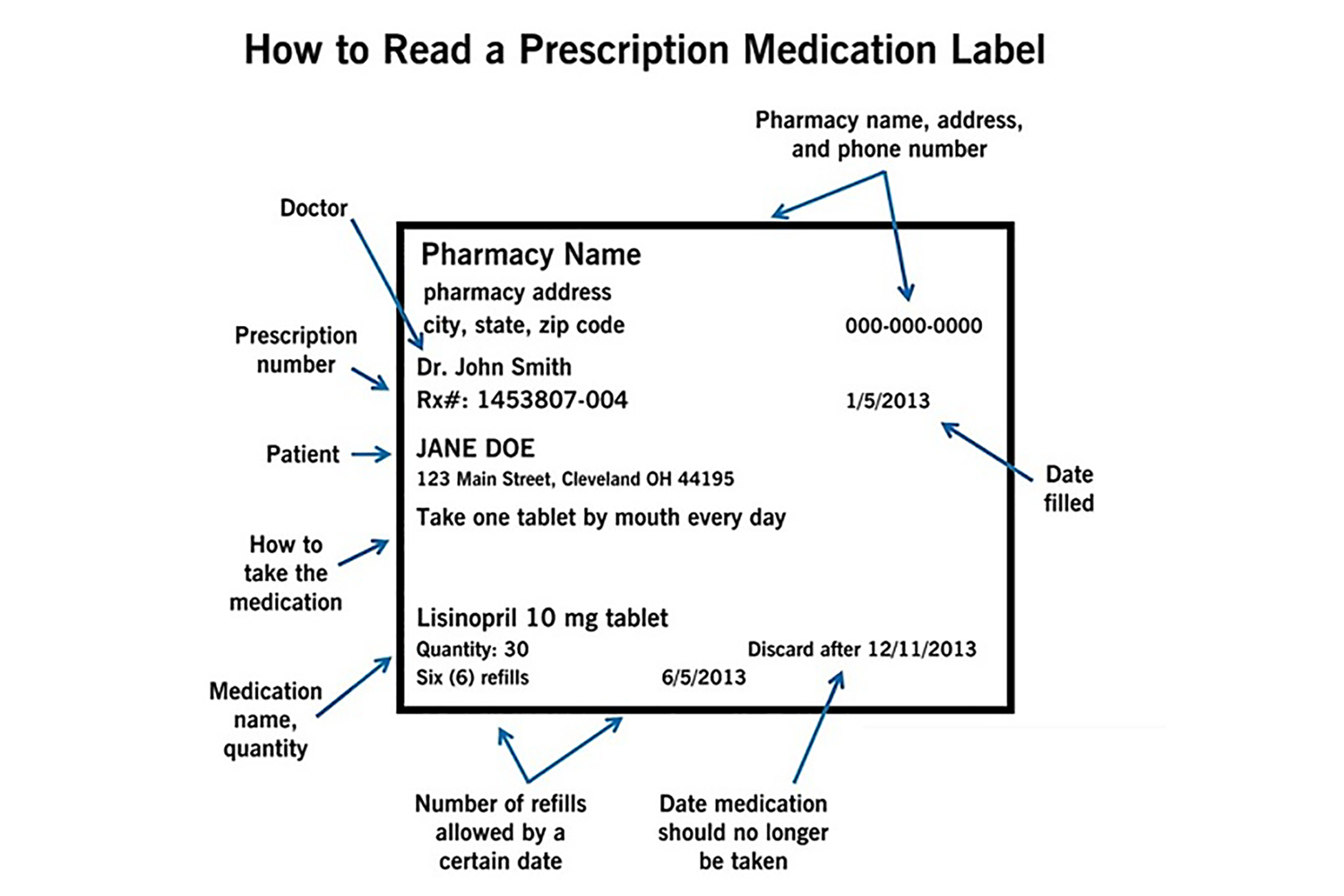
Prescription Medication Labels: How to Read - Cleveland Clinic Dec 22, 2020 · The label on your prescription medication tells you how to correctly take the medicine your healthcare provider has recommended for your treatment plan. It’s very important to understand the information on this label. By taking your medication correctly, you will have the best treatment results. CFR - Code of Federal Regulations Title 21 - Food and Drug... Jan 17, 2023 · The manufacturer or authorized distributor of record of a drug sample shall include on the label of the sample unit and on the outside container or packaging of the sample unit, if any, an identifying lot or control number that will permit the tracking of the distribution of each drug sample unit.
What is a lot number and how do I identify it? – Acme United ... Lot numbers have 2 parts: a letter, followed by a series of numbers. The letter corresponds to the month the product was manufactured: (A = January, B = February, C = March, D = April, E = May, F = June, G = July, H = August, I = September, J = October, K = November, L = December).
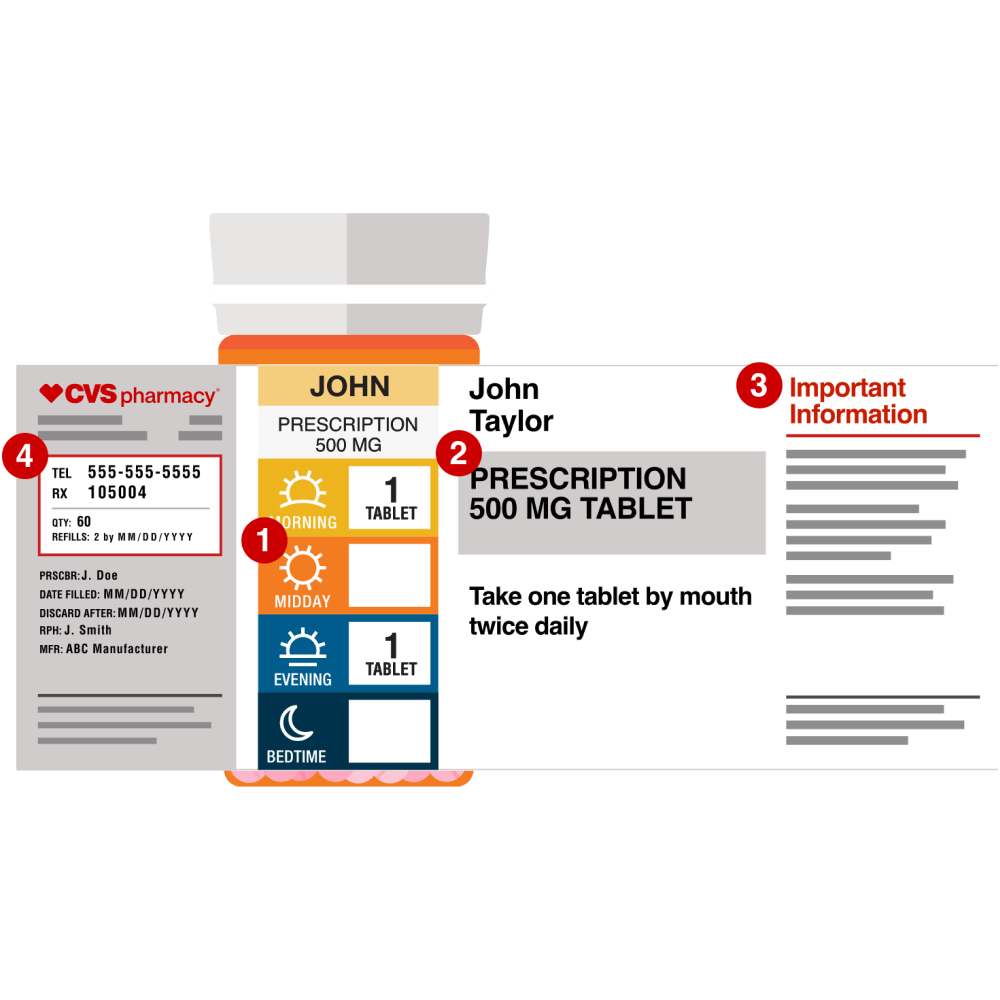
Lot number on medication label
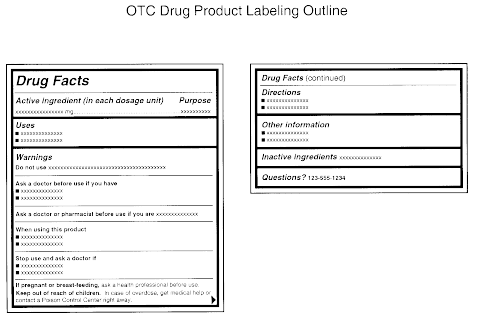
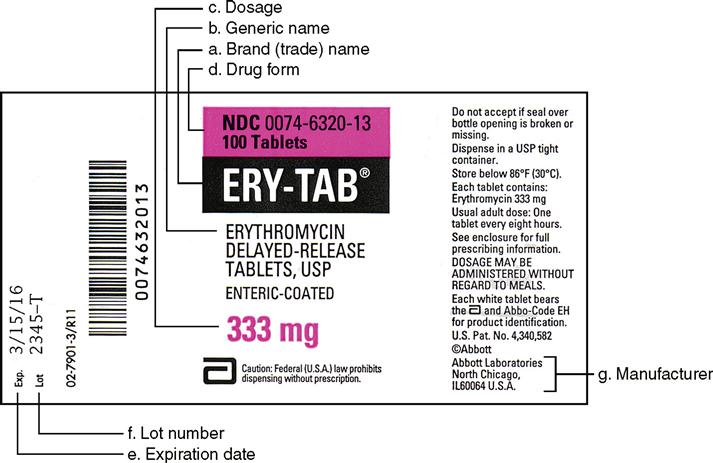
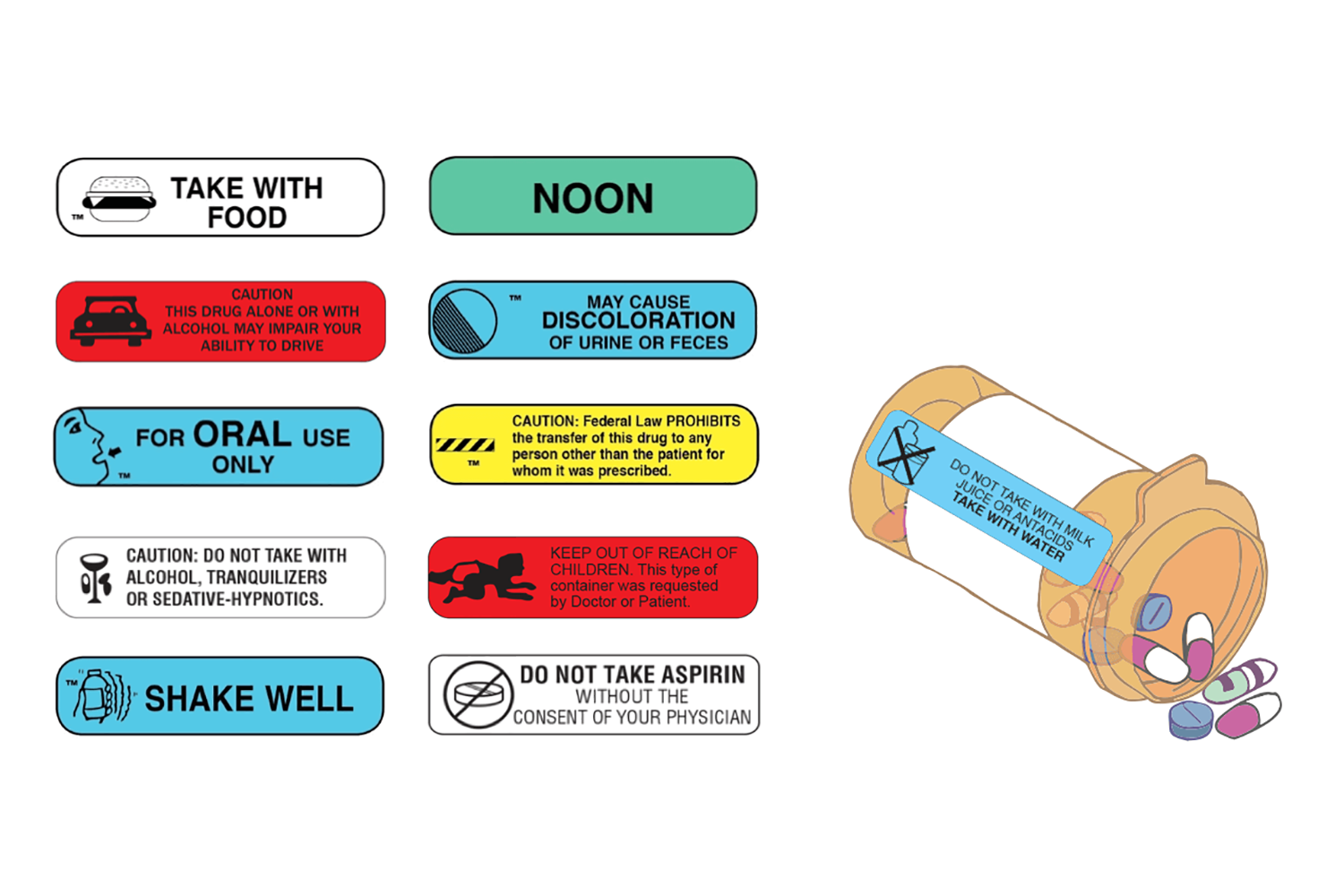
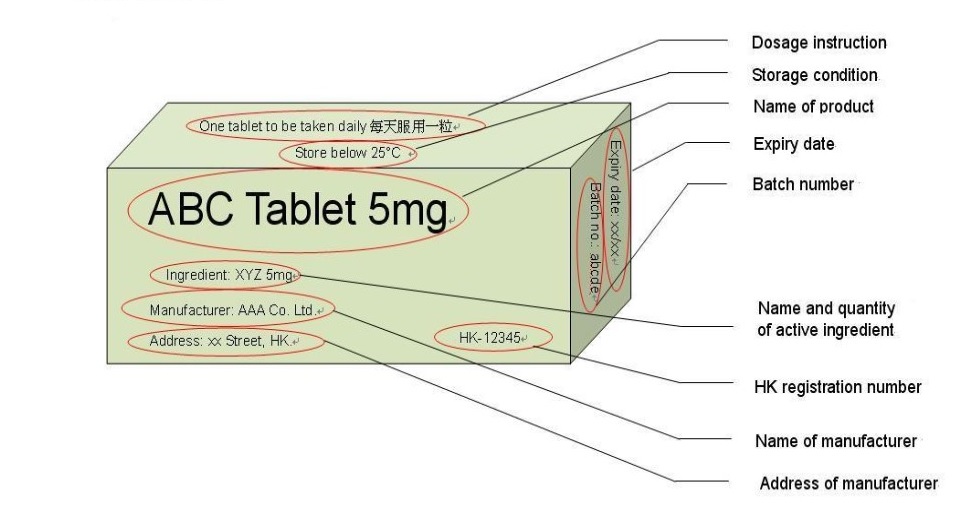
CHAPTER 20 LABELING MEDICATIONS AND EXPIRATION DATING A. UNIT DOSE MEDICATION – (Prepackaging) reference 64F-12.006 Minimum labeling to include: a) Name of drug (brand or generic or both) b) Strength c) Dosage Form d) Manufacturer e) Lot number f) Expiration date/beyond use date g) OR instead of (d) and (e) a control number which cross references to the manufacturer name and lot number Guidance Document: Labelling of Pharmaceutical Drugs for Human... Nov 1, 2013 · The dosage on consumer-available non-prescription drug product labels should state the number of tablets or capsules per dose, or the volume of product to be delivered (e.g., ml, teaspoon, tablespoon or where a calibrated dosing device should be used) and include the frequency of doses. CFR - Code of Federal Regulations Title 21 - Food and Drug... Jan 17, 2023 · The National Drug Code (NDC) number is requested but not required to appear on all drug labels and in all drug labeling, including the label of any prescription drug container furnished to a consumer. [40 FR 52002, Nov. 7, 1975, as amended at 81 FR 60212, Aug. 31, 2016]
Lot number on medication label. Labeling Information | Drug Products | FDA Nov 14, 2022 · For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the FDA’s Labeling Resources for Human... CFR - Code of Federal Regulations Title 21 - Food and Drug... Jan 17, 2023 · The National Drug Code (NDC) number is requested but not required to appear on all drug labels and in all drug labeling, including the label of any prescription drug container furnished to a consumer. [40 FR 52002, Nov. 7, 1975, as amended at 81 FR 60212, Aug. 31, 2016] Guidance Document: Labelling of Pharmaceutical Drugs for Human... Nov 1, 2013 · The dosage on consumer-available non-prescription drug product labels should state the number of tablets or capsules per dose, or the volume of product to be delivered (e.g., ml, teaspoon, tablespoon or where a calibrated dosing device should be used) and include the frequency of doses. CHAPTER 20 LABELING MEDICATIONS AND EXPIRATION DATING A. UNIT DOSE MEDICATION – (Prepackaging) reference 64F-12.006 Minimum labeling to include: a) Name of drug (brand or generic or both) b) Strength c) Dosage Form d) Manufacturer e) Lot number f) Expiration date/beyond use date g) OR instead of (d) and (e) a control number which cross references to the manufacturer name and lot number





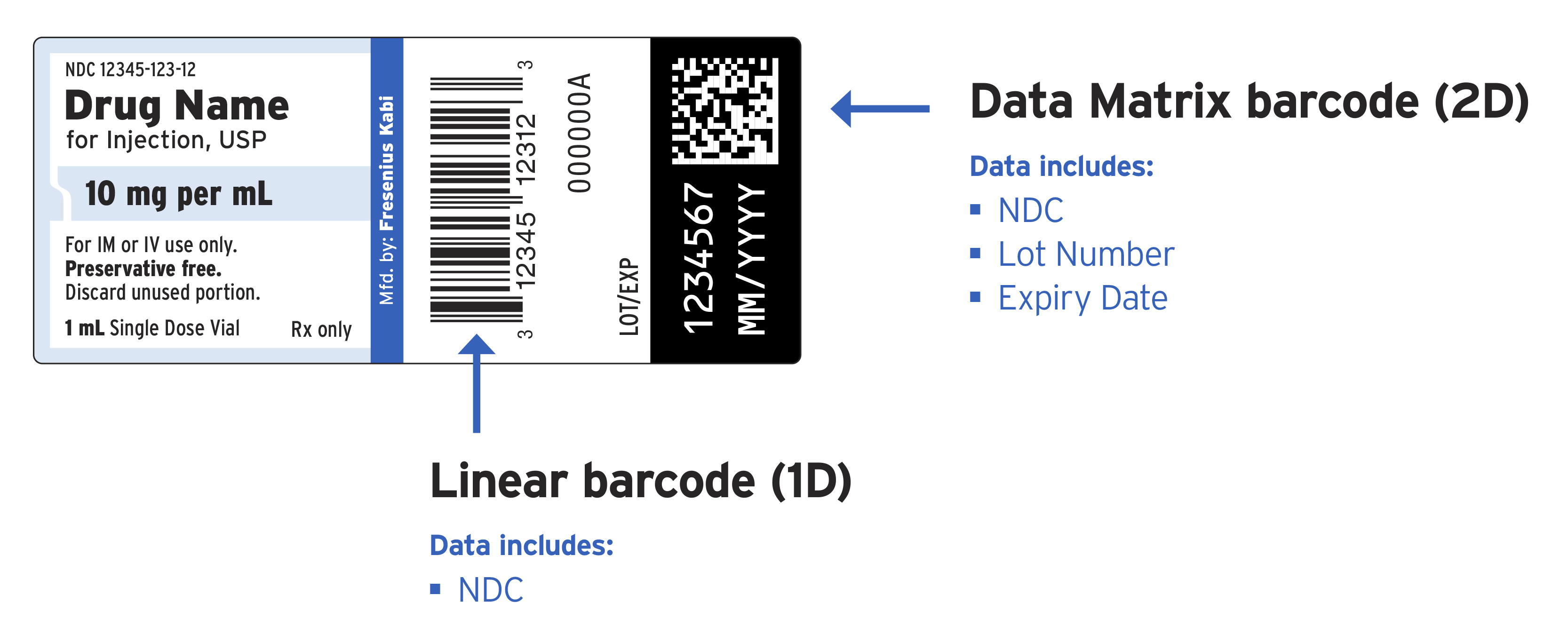

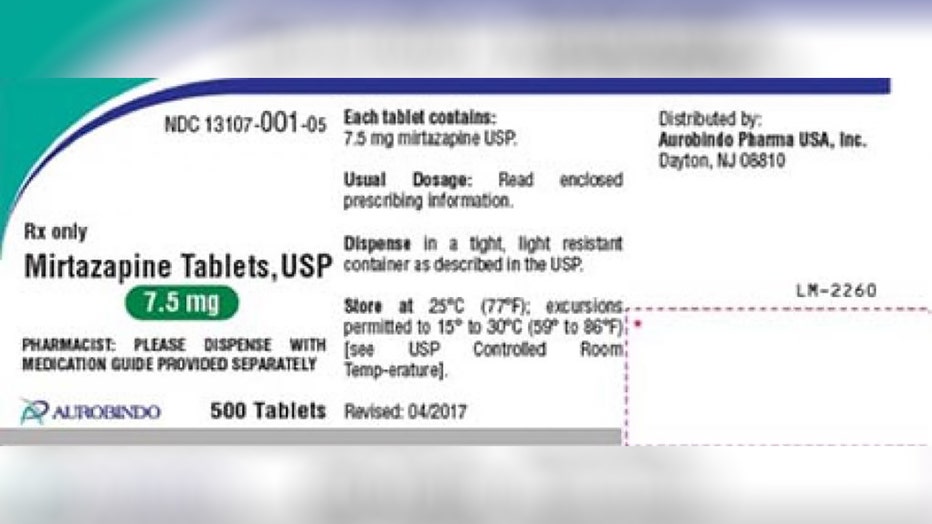



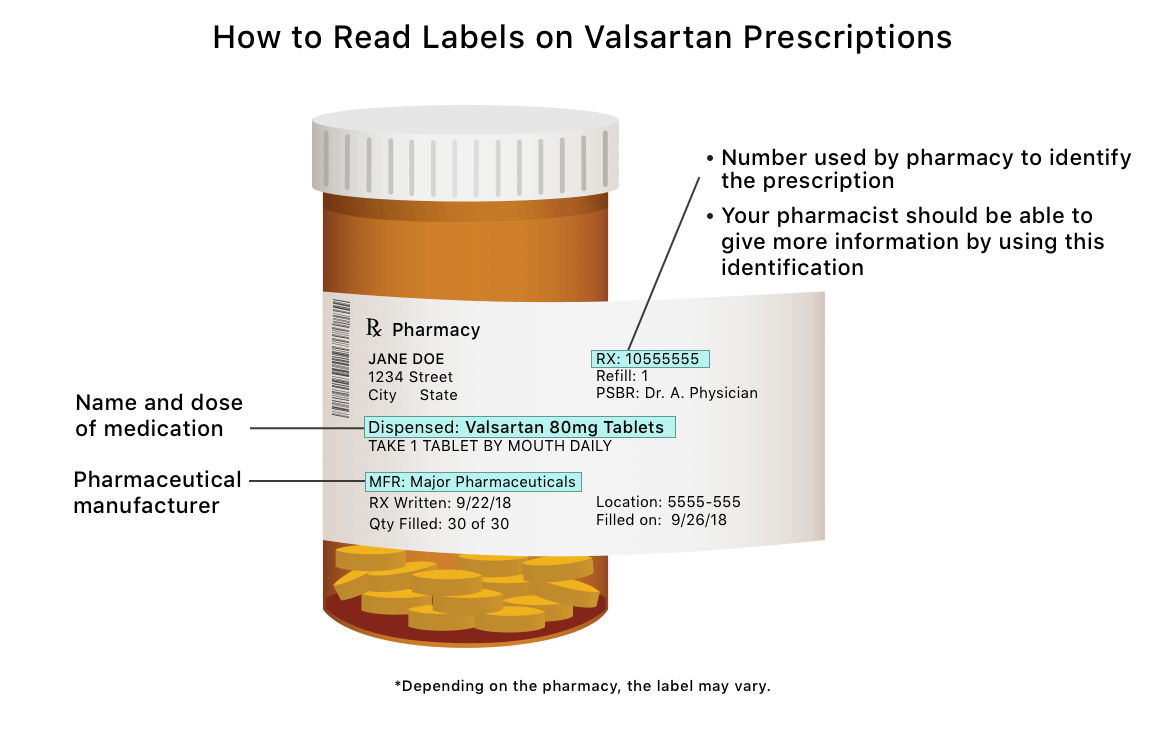

















/cloudfront-us-east-1.images.arcpublishing.com/gray/PZ2F7HUZENHLJBR2RERCIB67CE.jpg)


Post a Comment for "45 lot number on medication label"